LDAA Metabolite Calculator
Metabolite Analysis
Important: This tool is for educational purposes only. LDAA therapy should only be prescribed by a healthcare professional after proper testing and clinical evaluation.
When azathioprine stops working or starts damaging your liver, most doctors reach for a biologic. But there’s another path - one that’s been quietly changing outcomes for patients who don’t respond to standard doses. It’s called low-dose azathioprine with allopurinol, or LDAA. And for certain patients, it’s not just an alternative - it’s the only thing that brings them back to remission without the cost or risks of expensive biologics.
Why Azathioprine Alone Fails Some Patients
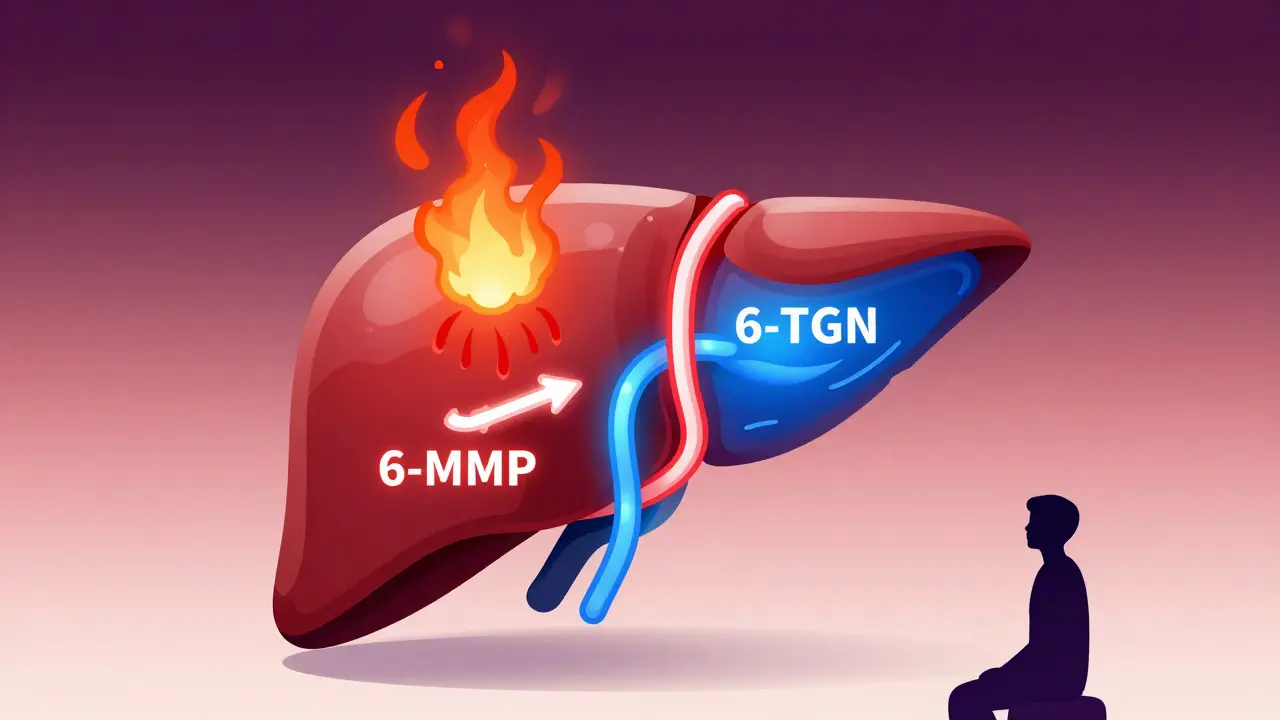
Azathioprine has been used since the 1960s to treat Crohn’s disease, ulcerative colitis, autoimmune hepatitis, and transplant rejection. But it doesn’t work the same way for everyone. The drug breaks down into 6-mercaptopurine (6-MP), which then splits into three different metabolites. Two of them matter most: 6-thioguanine nucleotides (6-TGN) and 6-methylmercaptopurine (6-MMP).6-TGN is the good guy. It gets built into your immune cells’ DNA and shuts down overactive inflammation. That’s what you want.
6-MMP is the problem. It builds up in your liver and causes toxic spikes in liver enzymes - nausea, jaundice, fatigue. Patients with high levels of 6-MMP are called “hypermethylators.” About 15-20% of IBD patients fall into this group. Their bodies convert too much azathioprine into 6-MMP instead of 6-TGN. So they get side effects without benefit.
Standard azathioprine doses - 1.5 to 2.5 mg per kg - just make it worse. Higher doses mean more 6-MMP. No wonder many patients quit the drug or end up with liver damage.
How Allopurinol Changes the Game
Allopurinol was designed for gout. It blocks an enzyme called xanthine oxidase, which normally breaks down purines. But here’s the twist: that same enzyme also helps destroy 6-MP before it can become 6-TGN. When you block xanthine oxidase with allopurinol, you don’t just lower uric acid - you redirect the entire metabolic pathway.Instead of 6-MP turning into 6-MMP (the bad metabolite), it gets shunted toward 6-TGN (the good one). Studies show this shift can reduce 6-MMP by 70-90% and boost 6-TGN levels by 2 to 5 times. That’s not a small tweak - it’s a full metabolic reset.
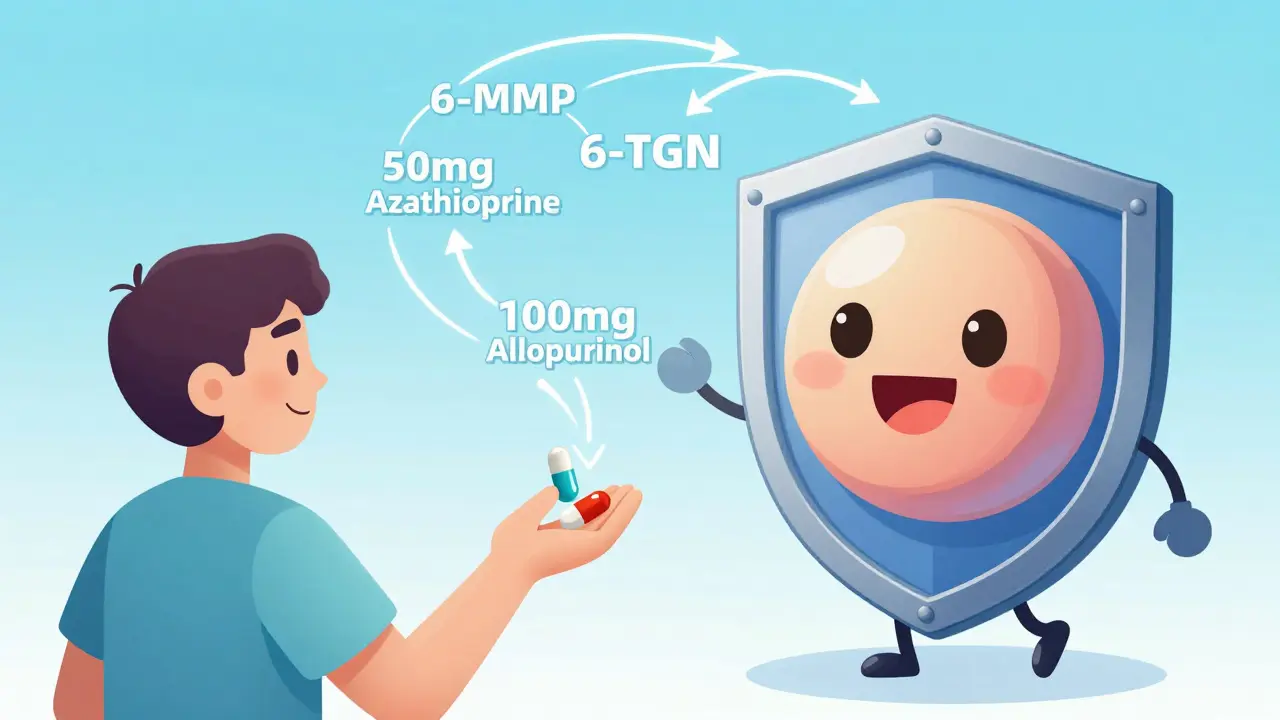
But here’s the catch: you can’t just add allopurinol to a full dose of azathioprine. That would flood your system with 6-TGN and crash your bone marrow. That’s why LDAA works only when azathioprine is cut to 25-33% of the usual dose - typically 50 mg per day instead of 150-200 mg.
Allopurinol is given at 100 mg daily. Together, this combo creates a new balance: enough 6-TGN to control inflammation, and almost no 6-MMP to hurt your liver.
Who Benefits Most From LDAA?
Not everyone is a candidate. LDAA is designed for one specific group: patients with high 6-MMP and low 6-TGN levels - the hypermethylators. This is confirmed through therapeutic drug monitoring (TDM), a blood test that measures metabolites in red blood cells.If your 6-MMP is above 5,700 pmol/8×10⁸ RBCs and your 6-TGN is below 230 pmol/8×10⁸ RBCs, you’re a perfect fit. Studies show these patients go from failing azathioprine to achieving remission in 65-75% of cases with LDAA. That’s nearly double the success rate of standard therapy.
It also helps patients who can’t tolerate azathioprine because of liver enzyme spikes. One 2018 study followed 42 patients with elevated ALT and AST. After switching to LDAA, 85-90% saw their liver enzymes return to normal within 8-12 weeks.
But LDAA won’t help patients with low TPMT activity - the enzyme that makes 6-MMP. These patients already make very little 6-MMP, but they’re at high risk of bone marrow suppression from any thiopurine. For them, LDAA is dangerous. Testing TPMT levels before starting is essential.
The Risks: Myelosuppression Is Real
LDAA isn’t risk-free. The biggest danger is myelosuppression - a drop in white blood cells, red blood cells, or platelets. Early reports in the 1980s linked azathioprine and allopurinol to fatal bone marrow failure. Those cases happened because doctors didn’t reduce the azathioprine dose.Today, with proper dosing and monitoring, the risk is manageable. But it’s still higher than with azathioprine alone. Studies show 25-40% of patients on unadjusted LDAA develop leukopenia. That’s why weekly blood counts for the first month are non-negotiable.
The nadir - the lowest point in white blood cell count - often hits between weeks 3 and 6. That’s when most patients get scared. But in 90% of cases, stopping azathioprine for 1-2 weeks and restarting at a slightly lower dose brings counts back up. Permanent discontinuation is rare if caught early.
LDAA is also not for people with severe kidney problems (creatinine clearance under 30 mL/min) or existing low blood counts. Allopurinol is cleared by the kidneys. If they’re not working well, the drug builds up and increases toxicity risk.
Real Patient Stories - Success and Caution
On Reddit’s r/IBD, users share their LDAA experiences. One patient, u/CrohnsWarrior2020, wrote: “After three years of failed Humira and high-dose azathioprine with liver enzymes through the roof, I switched to 50mg azathioprine + 100mg allopurinol. Liver enzymes normalized in 8 weeks. Been in remission for 14 months. No side effects.”Another, u/UlcerativeColitisNewbie, wasn’t so lucky: “Went on LDAA without monitoring. ANC dropped to 0.8. Hospitalized for fever and sepsis. Now I’m terrified of all immunosuppressants.”
The difference? Monitoring. The first patient had blood tests every week. The second didn’t. That’s the line between life-changing and life-threatening.
On professional review sites, gastroenterologists who use LDAA properly get 4.7 out of 5 stars. Those who avoid it entirely get 4.2. Patients don’t hate the drug - they hate being treated like a number.

How to Start LDAA - A Step-by-Step Guide
If you and your doctor decide LDAA is right for you, here’s what happens next:- Test first. Get your 6-TGN and 6-MMP levels checked. Confirm you’re a hypermethylator. Also check TPMT enzyme activity.
- Reduce azathioprine. Cut your dose to 25-33% of your previous amount. If you were on 150 mg, drop to 50 mg.
- Add allopurinol. Start 100 mg daily. No higher unless you have kidney issues.
- Monitor blood counts weekly. For the first 4 weeks, get a CBC every 7 days. After that, every 2 weeks for 3 months.
- Check metabolites at 4 weeks. Repeat the 6-TGN and 6-MMP test. Target: 6-TGN between 230-450 pmol/8×10⁸ RBCs. 6-MMP below 2,800 pmol/8×10⁸ RBCs.
- Adjust as needed. If 6-TGN is too low, increase azathioprine by 10-15 mg. If it’s above 450, reduce it. If 6-MMP is still high, double-check compliance or consider genetic factors.
Many clinics now use TDM as standard. But not all. If your doctor hasn’t heard of LDAA, bring the 2020 ECCO guidelines or the 2023 AGA technical review. They’re clear: LDAA is a valid, evidence-based option for the right patient.
Why LDAA Is Gaining Ground - But Still Underused
In Europe, 65% of IBD centers now use LDAA routinely. In the U.S., it’s only 35%. Why the gap? History. The FDA issued a black box warning in 1981 after deaths from unmonitored combinations. That warning never got updated to reflect modern protocols.Cost is another factor. A year of LDAA costs $1,200-$1,800. A biologic like Humira or Remicade? $30,000-$50,000. LDAA isn’t just safer for the liver - it’s 20 times cheaper.
And it’s expanding beyond IBD. A 2023 study in Hepatology showed 82% of autoimmune hepatitis patients who failed standard azathioprine went into remission with LDAA. That’s huge for a disease with few treatment options.
The future? Point-of-care metabolite testing. Two devices are in phase 3 trials - one that gives you your 6-TGN level in under an hour. Imagine walking into your GI’s office, getting a finger prick, and knowing your dose is right before you leave.
Final Takeaway: It’s Not a Last Resort - It’s a Precision Tool
LDAA isn’t for everyone. But for the 1 in 5 IBD patients stuck with high liver enzymes and no response to azathioprine, it’s often the only way forward. It doesn’t require a new drug. It doesn’t need a miracle. It just needs the right dose, the right test, and the right monitoring.If you’ve been told you’re “non-responsive” to azathioprine, ask about your metabolites. If your liver is failing but you still want to avoid biologics, ask about LDAA. It’s not experimental. It’s not fringe. It’s science - and it’s working.




Dan Alatepe
December 27, 2025 AT 21:31Bro. I was on 150mg azathioprine and my liver was screaming. Like, yellow eyes, nausea 24/7. Then my GI threw me LDAA like it was a secret weapon. 50mg + 100mg allopurinol. Liver enzymes dropped like a rock. No biologics. No $40k/year. I’m 18 months in and still kicking. They told me I’d need Humira. Nah. I got science and a $1500 bill instead. 🤝
Angela Spagnolo
December 27, 2025 AT 22:09i just read this whole thing and... wow. i had no idea this was even a thing. i’ve been on azathioprine for 5 years, and my dr never mentioned metabolites or testing. i’ve just been told ‘it’s not working’ and moved on. i’m going to print this out and bring it to my next appointment. thank you for writing this. i feel like i’ve been missing half the puzzle.
wendy parrales fong
December 28, 2025 AT 21:12This is beautiful. Real medicine isn’t about the newest, flashiest drug. It’s about understanding how your body works and working with it. Azathioprine isn’t broken. It’s just being used wrong for some people. Allopurinol isn’t magic. It’s a tool. And when you pair it with the right dose and testing? It’s like tuning a guitar instead of smashing it. People deserve this kind of care. Not just a prescription and a shrug.
Jeanette Jeffrey
December 29, 2025 AT 09:45Oh great. Another ‘miracle combo’ that requires 7 blood tests a month and still kills people if you blink wrong. Look, I get it. You’re excited. But this isn’t ‘precision medicine’-it’s just a band-aid on a bullet wound. And the fact that it’s 20x cheaper means pharma doesn’t make money. That’s why it’s underused. Not because doctors are dumb. Because the system is rigged.
Shreyash Gupta
December 31, 2025 AT 07:19Wait so if you have high 6-MMP you can’t just take less azathioprine? You need allopurinol? That’s wild. I thought it was just ‘dose adjustment’. I’ve been reducing mine for months and still got liver spikes. Maybe I was doing it wrong. 🤔
Ellie Stretshberry
January 1, 2026 AT 04:18my cousin tried this and it saved her. she was on the verge of a colectomy. now she’s hiking. no biologics. no hospital stays. just a tiny pill and weekly blood tests. i cried reading this. people need to know this exists. it’s not a last resort. it’s a second chance.
Zina Constantin
January 2, 2026 AT 06:00As someone who grew up in a family of doctors, I’ve seen how lazy medicine works. ‘Try this drug. If it fails, try the next.’ No testing. No nuance. LDAA is the antidote to that. It’s not flashy, but it’s elegant. It’s using what we already have, smarter. And the fact that it’s underused in the U.S.? That’s a failure of education-not science.
Sarah Holmes
January 3, 2026 AT 13:00This is dangerously irresponsible. You’re advocating for a regimen that carries a 40% risk of leukopenia without emphasizing that most patients will need to be hospitalized if they skip monitoring. This isn’t ‘science.’ It’s a gamble dressed in clinical jargon. If your doctor doesn’t have a dedicated hematologist on standby, don’t touch this. People die from this. Don’t romanticize it.
Jay Ara
January 3, 2026 AT 14:38my dr told me i was a hypermethylator. i was scared. but after 3 weeks of LDAA? my energy came back. no more nausea. no more liver pain. i just wish i’d known this 4 years ago. you dont need a miracle drug. you need to know your body. and your dr needs to listen.
Michael Bond
January 4, 2026 AT 23:02LDAA works. But only if you test. And monitor. And adjust. That’s it.
Kuldipsinh Rathod
January 5, 2026 AT 16:55my uncle did this in india. no fancy labs. just a basic blood test every two weeks. he’s been in remission for 3 years now. cost? less than $50 a month. if this works in a village clinic, why isn’t it everywhere?