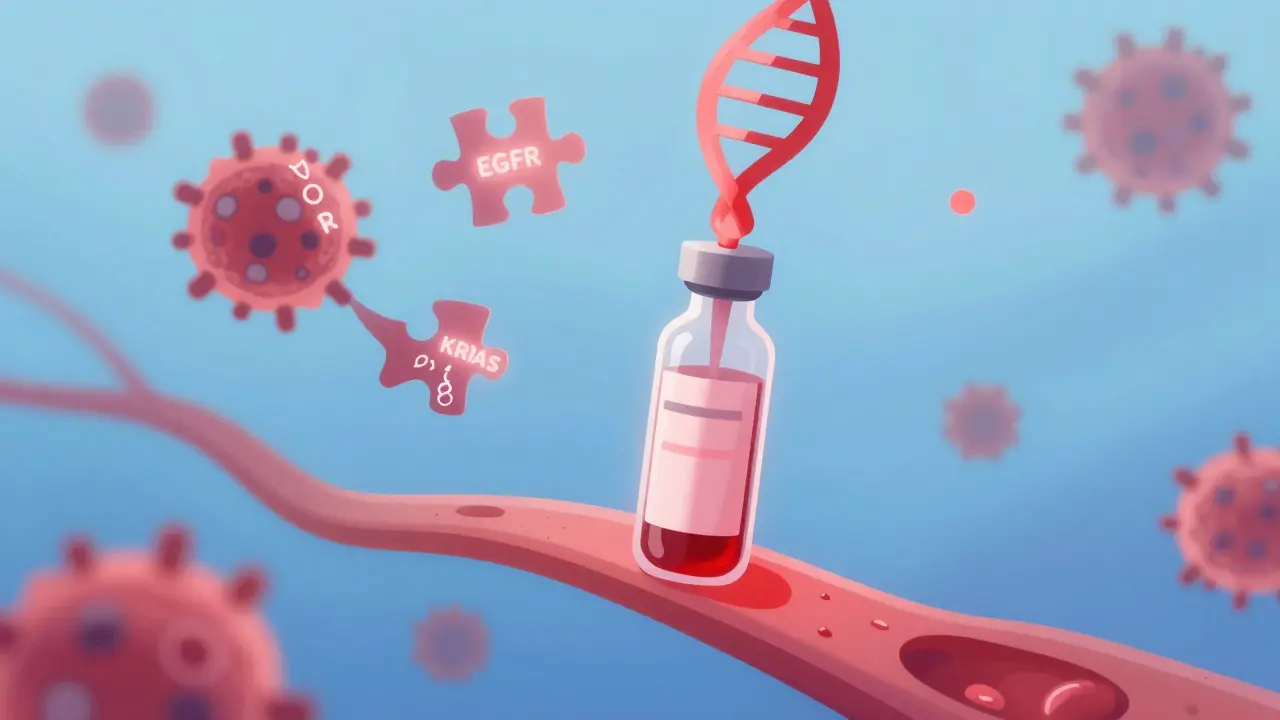
Imagine knowing if your cancer treatment is working - not weeks after a scan, but in days. Not by poking into your lung or liver with a needle, but by drawing a single vial of blood. That’s not science fiction. It’s circulating tumor DNA - or ctDNA - in action.
What Is Circulating Tumor DNA?
When cancer cells die, they don’t just disappear. They break apart and spill bits of their DNA into the bloodstream. These fragments are called circulating tumor DNA, or ctDNA. Unlike healthy DNA floating around, ctDNA carries the exact genetic mistakes that made the tumor grow - mutations in genes like EGFR, KRAS, or BRAF. It’s like finding a wanted poster in the river, only this poster is made of the tumor’s own genetic code. It’s not the only thing in the blood - there are also circulating tumor cells and tumor-derived vesicles - but ctDNA is the most reliable marker. It’s stable, measurable, and directly tied to the tumor’s biology. And because it’s released continuously, it gives doctors a real-time window into what the cancer is doing, right now.Why Liquid Biopsy Beats Traditional Biopsies
Traditional tissue biopsies are invasive. A lung tumor? You need a needle guided by CT. A brain tumor? Surgery. And even then, you’re only seeing one piece of the tumor. Cancers aren’t uniform. One part might have a mutation that makes it resistant to a drug, while another part doesn’t. A single biopsy can miss that entirely - up to 30% of the time. Liquid biopsy solves that. A blood draw captures DNA from all parts of the tumor, everywhere it’s spread. It’s not just safer - it’s smarter. And because it’s quick and painless, you can do it again. And again. Every few weeks. That’s impossible with surgery. In metastatic cancer, where tumors are all over the body, tissue biopsies become impractical. About 20-30% of patients simply don’t have enough tissue left to test. Liquid biopsy steps in. At MD Anderson Cancer Center, doctors now use it to avoid repeat tissue biopsies in 25-30% of cases. Patients breathe easier. Doctors get more data.How Is ctDNA Detected?
Detecting ctDNA is like finding one red marble in a bathtub full of white ones. The mutant DNA might make up less than 0.1% of all DNA in the blood. That’s why the tools have to be incredibly precise. The most common methods:- Digital droplet PCR (ddPCR) - splits blood samples into thousands of tiny droplets. Each droplet holds a single DNA molecule. If it’s mutated, it lights up. Can detect one mutant in 10,000 normal molecules.
- Next-generation sequencing (NGS) - reads millions of DNA fragments at once. Can screen dozens of cancer genes in one test.
- Methylation analysis - looks at chemical tags on DNA. Cancer DNA has different tagging patterns than healthy DNA. This method boosts detection by 20-30% for early-stage tumors.
- Nanopore sequencing - reads DNA strands as they pass through a tiny pore. Works with both short and long fragments, giving more complete data.

When Is Liquid Biopsy Used?
It’s not for everyone. But for certain cancers and stages, it’s becoming standard.- Monitoring treatment response - If ctDNA levels drop after chemo or immunotherapy, the treatment is working. If they rise, resistance is developing - often months before a scan shows it.
- Detecting minimal residual disease - After surgery, if ctDNA is still present, the cancer is likely hiding. Studies show it can predict recurrence 6-11 months before imaging does.
- Guiding targeted therapy - In lung cancer, ctDNA found EGFR mutations in 92% of cases where tissue wasn’t available. That means patients got the right drug faster.
- Identifying resistance mutations - A patient on osimertinib for EGFR-positive lung cancer? ctDNA can spot the T790M mutation that makes the drug stop working. Doctors switch treatment before the tumor grows.
- Early detection in high-risk people - People with inherited cancer syndromes or strong family history can get screened yearly. Methylation-based tests are especially promising here - they can catch cancer before symptoms appear.
Where It Falls Short
It’s powerful - but not perfect. Early-stage cancers shed very little ctDNA. For stage I tumors, detection rates are only 50-70%. For stage IV, it’s 80-90%. That’s why it’s not yet used for population-wide screening. Some cancers barely release DNA. Brain tumors, certain lymphomas, and slow-growing tumors often give false negatives. Blood tests might say “no cancer,” but the tumor is still there. Then there’s noise. Not every mutation in the blood comes from cancer. Aging causes harmless mutations in blood cells - called clonal hematopoiesis. It happens in 10-15% of people over 65. A test might flag it as cancer. That’s a false alarm. And variants of unknown significance? They show up in 15-20% of reports. A mutation is found - but no one knows if it matters. That leaves doctors and patients stuck in uncertainty.
What’s Next?
The field is moving fast. Researchers are combining ctDNA with fragmentomics - studying the size and shape of DNA fragments - to spot cancer patterns no one’s seen before. At MD Anderson, AI is being trained to recognize these patterns. Early results suggest a 15-20% boost in accuracy. Methylation profiling is the next big leap. Cancer changes how DNA is tagged before it’s even mutated. That means methylation tests could detect cancer before it’s visible on a scan. Trials are already underway for multi-cancer early detection (MCED) tests. Standardization is the biggest hurdle. Different labs use different blood tubes, processing times, and machines. That leads to 25% variation in results between centers. Until everyone follows the same protocol, results won’t be fully reliable. But progress is real. By 2030, the global liquid biopsy market is expected to hit $19.5 billion. More hospitals are offering it. Community clinics are catching up. And patients? They’re getting less invasive care, fewer scans, and more personalized treatment.What This Means for You
If you or someone you know has advanced cancer - especially lung, colorectal, or breast - ask about liquid biopsy. It’s not a replacement for imaging, but it’s a powerful complement. It tells you what the tumor is doing at the molecular level, not just how big it looks on a scan. It’s not magic. It won’t cure cancer. But it gives you more control. More information. More time to act before things get worse. The future of cancer care isn’t just about stronger drugs. It’s about smarter monitoring. And liquid biopsy - powered by circulating tumor DNA - is leading the way.Can liquid biopsy replace a tissue biopsy completely?
No. Tissue biopsy is still the gold standard for initial diagnosis because it gives the full picture of tumor structure, grade, and surrounding tissue. Liquid biopsy is best used after diagnosis - for monitoring, detecting resistance, or when tissue is unavailable. In some cases, like advanced lung cancer, guidelines allow liquid biopsy as a first test if tissue can’t be safely obtained.
How often should ctDNA be tested during treatment?
It depends on the cancer type and treatment phase. During active treatment (like chemo or targeted therapy), testing every 4 to 8 weeks is common. After treatment ends, during surveillance, testing every 3 to 6 months helps catch recurrence early. Some centers test at each clinic visit if the patient is on a targeted drug with known resistance risks.
Is liquid biopsy covered by insurance?
In the U.S., Medicare and most private insurers cover FDA-approved liquid biopsy tests for advanced cancers when used according to guidelines - especially for lung, colorectal, and breast cancer. Coverage varies for early detection or non-approved uses. Always check with your provider and the testing lab.
Can liquid biopsy detect cancer before symptoms appear?
Possibly - but not reliably yet. Current tests work best in people already diagnosed with cancer or at very high risk (like those with BRCA mutations). Multi-cancer early detection tests using methylation and fragmentomics are in clinical trials and show promise for catching cancers like pancreatic or ovarian before they spread. But they’re not ready for routine use in the general population.
What if the liquid biopsy result is negative but I still have symptoms?
A negative result doesn’t rule out cancer, especially in early stages or low-shedding tumors. Always follow up with imaging or other tests if symptoms persist. Liquid biopsy is a tool, not a final answer. Doctors use it alongside scans, physical exams, and tumor markers - never alone.





Blow Job
December 24, 2025 AT 16:05This is wild. I had no idea we could catch cancer changes just from a blood draw. Feels like something out of a movie, but it’s real-and it’s saving people from unnecessary surgeries.
Rachel Cericola
December 26, 2025 AT 11:51Let me tell you, as someone who’s watched a close family member go through metastatic lung cancer, this isn’t just science-it’s survival. Traditional biopsies were brutal. Needle in the lung? Under CT guidance? He cried after that one. Liquid biopsy changed everything. We went from waiting weeks for scans to getting results in 72 hours. When his ctDNA dropped after the first round of osimertinib, we knew it was working before the tumor shrank on the scan. And when it started rising again? We switched meds before he felt worse. That’s power. That’s hope. And yes, it’s not perfect-false negatives happen, especially in early stages-but when you’re out of options, this is the lifeline you pray for. The fact that it’s now in NCCN guidelines? Long overdue. We need this everywhere, not just in big hospitals. Community oncologists need training, insurance needs to stop playing games, and labs need standardization. Right now, you get different results depending on which tube they use. That’s unacceptable. We’re talking about people’s lives here. This tech deserves better than patchwork implementation.
Dan Gaytan
December 27, 2025 AT 18:37YES. 🙌 I work in a clinic and we just started offering Guardant360 for our lung cancer patients. The relief on their faces when they hear ‘no needle, no CT, just a blood draw’? Priceless. One guy said, ‘I’d rather get a root canal than another biopsy.’ Now he gets tested every 6 weeks like clockwork. It’s not magic, but it’s the closest thing we’ve got.
Chris Buchanan
December 28, 2025 AT 13:24So we’re replacing surgery with… a fancy blood test? Cool. I’m sure the pharma companies love this. More tests = more revenue. Who needs real diagnosis when you can just keep pumping out $1,200 blood panels? 🤔
Jeffrey Frye
December 29, 2025 AT 01:03lol at the ‘gold standard’ bs. tissue biopsies are a relic. they’re like using a typewriter to write a novel. you get one shot, it’s messy, and half the time you’re just guessing. ctDNA is the future. they’re just scared to admit it because surgeons don’t get paid for blood draws. and don’t even get me started on ‘clonal hematopoiesis’-that’s just the system covering its ass when it gets a false positive. they call it noise, i call it censorship.
Andrea Di Candia
December 29, 2025 AT 21:56I love how this isn’t just about tech-it’s about dignity. Imagine being told you need another invasive procedure, and instead, you just roll up your sleeve. No prep. No recovery. No fear of puncturing a lung. It’s not just accurate-it’s humane. And honestly? That matters more than we admit. Cancer already takes so much. The least we can do is make the monitoring part less of a war zone. I’m not saying it’s perfect, but it’s a step toward treating people like humans, not just data points. And that? That’s worth celebrating.
bharath vinay
December 31, 2025 AT 04:54They say it’s ‘revolutionary’ but they’ve been saying that about cancer cures since the 70s. This is just another distraction. The real cause? 5G radiation, glyphosate, and mRNA vaccines altering your DNA. They want you to believe a blood test can fix it so you don’t ask why your food is poisoned or why your phone is frying your cells. Wake up.
claire davies
December 31, 2025 AT 20:31As someone who’s lived in both the US and the UK, I’ve seen the gap. Over here, NHS waits for tissue biopsies to clear bureaucratic red tape-sometimes months. Meanwhile, in the States, folks get liquid biopsy results in under a week. It’s not just about the science-it’s about systems. We need global protocols. Why can’t we have one standard for blood collection tubes? Why does every lab use a different extraction kit? It’s madness. And don’t get me started on the cost. In Australia, it’s covered under PBS for advanced cases. In the UK? Only if you’re lucky. This tech should be a human right, not a privilege tied to your zip code.
Usha Sundar
January 1, 2026 AT 12:15My mom’s test came back negative. Scan showed a 3cm tumor. They told her to wait. Now she’s in hospice. Don’t trust the blood test.
Wilton Holliday
January 2, 2026 AT 06:02Just had a patient on osimertinib for 14 months. ctDNA was flat. Then-boom-T790M spike. We switched to amivantamab before she had a single new symptom. That’s the power. This isn’t just monitoring-it’s prevention. And yeah, it’s expensive. But so is treating stage IV cancer after it’s been ignored for six months because ‘the scan looked fine.’ This saves money in the long run. Plus, less trauma. Less fear. More control. That’s priceless.
Delilah Rose
January 2, 2026 AT 18:28I’ve been reading everything I can about ctDNA since my sister’s diagnosis. It’s fascinating how the size and shape of the DNA fragments-fragmentomics-can reveal cancer patterns even before mutations show up. It’s like the tumor leaves behind footprints in the blood, not just fingerprints. And methylation? That’s the holy grail. Cancer changes how DNA is tagged before it even mutates. So you’re catching it before it’s cancer. That’s next-level. I know it’s not ready for everyone yet, but the fact that trials are already testing this for pancreatic and ovarian cancers? That gives me hope. Maybe one day, we’ll catch it before it’s even a tumor. Just a whisper in the blood.
Andy Grace
January 3, 2026 AT 03:18Interesting stuff. I work in a rural clinic. We don’t have access to NGS or ddPCR. Our lab sends samples out. Turnaround is 3 weeks. That’s useless for monitoring. Until this tech is affordable and fast everywhere-not just in fancy cancer centers-it’s not a solution. It’s a luxury.
Blow Job
January 3, 2026 AT 07:06That’s why I’m pushing for community lab partnerships. My cousin’s oncologist got a deal with a national lab-same test, half the cost, results in 5 days. We need more of that. Not everyone lives near MD Anderson.
Joseph Manuel
January 3, 2026 AT 12:34While the clinical utility of ctDNA is well-documented in metastatic NSCLC, the sensitivity thresholds for early-stage disease remain suboptimal. Furthermore, the analytical variability across platforms-particularly in pre-analytical sample handling-introduces significant heterogeneity in clinical decision-making. Until standardized reference materials and ISO-certified protocols are universally adopted, widespread implementation remains premature.
Chris Buchanan
January 5, 2026 AT 07:08So… we’re paying $1,500 to avoid a $500 biopsy? And you call that progress? Next thing you know, they’ll charge us $200 to breathe.