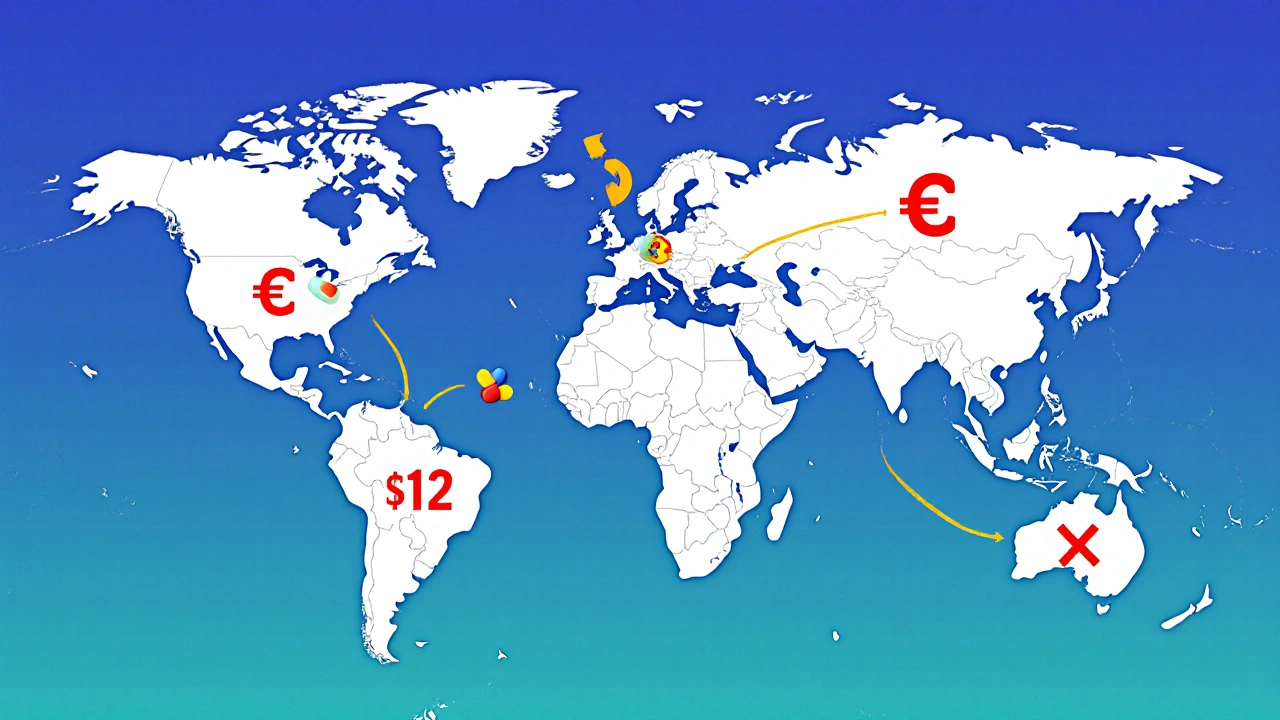
How Countries Use International Reference Pricing to Control Generic Drug Costs
Imagine you walk into a pharmacy in Berlin and pay €0.50 for a generic blood pressure pill. Two weeks later, you’re in Madrid and pay the same amount. But in New York, the exact same pill costs $12. Why the difference? The answer isn’t quality, manufacturing, or ingredients. It’s international reference pricing - a system used by most high-income countries to set what they pay for generic medicines by looking at what other countries pay.
International reference pricing (IRP) isn’t new. It started in the 1980s when European governments saw pharmaceutical spending spiral out of control. Italy was the first to formalize it in 1984, followed by Spain and Portugal. Today, 34 out of 38 high-income countries use some form of IRP, and 28 of 32 European nations apply it specifically to generic drugs. The goal is simple: keep prices low without cutting access. And for generics - drugs that are chemically identical to brand-name versions but cost far less - IRP has become the main tool to drive down prices.
How IRP Actually Works for Generic Drugs
There’s a big misunderstanding about how IRP works. Many think countries just copy the lowest price in the world. That’s not true. Most countries use what’s called internal reference pricing for generics - not external.
Internal reference pricing means grouping together all generic drugs that treat the same condition - like all 10mg lisinopril tablets - and setting one reimbursement price. If a drug in that group costs more than the lowest-priced version, the government only pays up to that lowest price. The pharmacy or patient pays the difference. This pushes manufacturers to compete on price within the group.
Germany’s system, called AMNOG, is a good example. Since 2011, it sets reimbursement for generic groups at the lowest price in the group plus a 3% margin. That means if five companies sell the same generic, and one sells it for €0.20, the others can’t get more than €0.21 from the government. The result? Prices drop fast, and patients still get the same medicine.
Other countries like the Netherlands mix IRP with tendering - where the government invites bids from manufacturers and picks the cheapest. That’s how Dutch generic prices ended up 65-85% lower than the original branded versions.
Which Countries Are Used as Price References?
When countries use external reference pricing (looking at prices in other nations), they don’t pick random countries. They pick similar ones - economically, culturally, and medically.
Western European countries typically look at France, Germany, Italy, Spain, and the UK. Eastern European nations often use Austria, Germany, and the Netherlands. Switzerland does something different: it takes two-thirds of the average price from its reference countries and adds one-third based on its own domestic prices. That way, it doesn’t get dragged down by cheaper neighbors but still stays competitive.
The number of reference countries matters. Studies show that using 5 to 7 countries gives the best balance: big enough to get meaningful data, small enough to avoid chaos. Countries using 10 or more reference countries saw only a 31% price drop - but a 12% increase in medicine shortages. Why? Because when you compare too many markets, you end up chasing the lowest price, which often means manufacturers can’t make a profit.
Why IRP Works Better for Generics Than Brand Drugs
For brand-name drugs, IRP is often used to cap the maximum price a company can charge. But for generics, it’s not about capping - it’s about creating competition.
Here’s the key difference: brand-name drugs are unique. Generics are interchangeable. That’s why 24 of 27 EU countries use internal reference pricing for generics, but only 12 use external reference pricing. Internal pricing works because the drugs are the same. There’s no innovation to protect. The goal is to make sure no one pays more than necessary.
Some countries, like the United States, don’t use IRP at all for federal programs. But even there, states like Colorado have tried it for Medicaid generics - and saw 12-15% savings. Canada doesn’t use IRP for generics either. Instead, provinces run their own tendering systems. So IRP isn’t the only way to control prices - but it’s the most common in Europe.

The Hidden Costs: Shortages, Quality Concerns, and Market Exit
IRP isn’t perfect. The biggest problem? When prices get too low, companies stop making the drugs.
During Greece’s financial crisis, the government forced generic prices down sharply. By 2015, 37% of generic medicines had shortages. Pharmacies couldn’t stock them because manufacturers couldn’t cover costs. The same thing happened in Portugal in 2019, when 22 generic products disappeared from the market after pricing rules made them unprofitable.
Manufacturers aren’t just cutting corners. Complex generics - like inhalers, injectables, or extended-release tablets - cost almost as much to develop as new drugs. But IRP systems often treat them the same as simple pills. A 2020 FDA study found a 17% drop in new complex generic applications in countries with strict IRP. That means fewer options for patients who need them.
Patients notice too. A 2021 OECD survey found that while 78% of Europeans were happy with generic substitution, 34% worried about quality differences between the cheapest version and more expensive ones. Pharmacists in Spain say 63% of them have run out of the lowest-priced reference product, forcing them to switch patients to another brand - even if the patient prefers the original.
How Countries Are Fixing the Problems
Some countries are changing how they do IRP to avoid the downsides.
France launched a new system in January 2023 called dynamic reference pricing. Instead of setting prices once a year, it adjusts them quarterly based on market share. If a cheaper generic starts selling more, its price gets lowered. If it’s losing ground, the price doesn’t drop as much. Early results show 8.2% extra savings without major shortages.
The European Commission is testing a new European Reference Pricing Platform, launched in April 2023. It’s a shared database where seven countries pool data on 15 generic drugs. The goal? To make pricing more transparent and prevent countries from undercutting each other. If it works, it could expand to 100 drugs by 2025.
Experts like Professor Panos Kanavos from LSE say the sweet spot is using 5-7 reference countries and setting prices based on the median, not the lowest. That avoids the pricing spiral - where one country cuts prices, others follow, and everyone ends up with shortages.
What This Means for Patients and Manufacturers
For patients, IRP usually means lower out-of-pocket costs and more access to generics. In Spain, generic substitution rates jumped from 52% in 2010 to 89% today because of internal reference pricing. That’s good for affordability.
But it’s not all win-win. Generic drugmakers like Teva and Sandoz say IRP systems have cut their European revenues by 9% even as sales volume grew by 15%. That’s because profit margins are razor-thin. Companies that focus on quality, consistency, and complex generics are surviving. Those that only compete on price are disappearing.
Hospitals and pharmacies benefit too. Germany’s system reduced administrative work by 37% because pharmacists don’t have to negotiate prices for every single generic - the government already set the reimbursement rate. That saves time and money.

What’s Next for International Reference Pricing?
IRP isn’t going away. In fact, IQVIA predicts that by 2027, 65% of European generic prices will be set by reference systems - up from 58% in 2022. But the systems are evolving.
The OECD now recommends tiered reference groups - where simple generics are priced one way, and complex ones are priced differently based on development cost. That’s a big shift. It means the system is starting to recognize that not all generics are the same.
Future systems will likely combine IRP with value-based pricing - where the price reflects not just cost, but how well the drug works in real life. For example, a generic that reduces hospital readmissions might get a higher reimbursement than one that doesn’t.
For now, the balance remains delicate: low prices for patients, sustainable profits for manufacturers, and steady supply for pharmacies. The countries that get this right - like the Netherlands, Germany, and now France - are the ones keeping generics affordable without breaking the system.
What You Should Know if You’re a Patient or Prescriber
- Generic drugs are legally required to be identical in active ingredients, dosage, and effectiveness - no matter the price.
- If your pharmacy switches your generic brand, it’s usually because of IRP rules, not your doctor’s choice.
- Don’t assume the cheapest generic is the worst. Many are made by the same companies that make branded drugs.
- If you can’t get your usual generic, ask your pharmacist if there’s another in the same reference group. They’re therapeutically equivalent.
- Shortages happen - but they’re usually temporary. Talk to your doctor if you’re affected.
What is international reference pricing for generic drugs?
International reference pricing (IRP) is a system where governments set the price of generic medicines by comparing prices in other countries. Instead of letting manufacturers charge what they want, the government uses prices from a group of similar countries to determine how much it will pay - and often, how much patients pay too. It’s used mainly in Europe to keep generic drug costs low.
Does the U.S. use international reference pricing for generics?
No, the U.S. federal government does not use international reference pricing for generics. However, some states like Colorado have experimented with it for Medicaid prescriptions and saw 12-15% cost savings. Most U.S. generic prices are set by market competition and pharmacy benefit managers, not government reference pricing.
Why do some generic drugs disappear from pharmacies?
When reference pricing forces prices too low, manufacturers can’t make enough profit to cover production costs - especially for complex generics like inhalers or injectables. This leads to market exit. Greece and Portugal saw dozens of generics disappear during strict IRP periods. It’s not about quality - it’s about economics.
Is the cheapest generic always the best choice?
Yes, for most patients. All approved generics must meet the same strict standards for safety and effectiveness as brand-name drugs. The cheapest one is usually the best choice because it’s the same medicine, just cheaper. But if you have a reaction or notice a difference, talk to your doctor - it could be an inactive ingredient issue, not the active drug.
How often do countries update generic prices under IRP?
Most countries update prices once a year. Some, like France, now update quarterly using dynamic systems. During crises, like Greece’s financial collapse, updates happened every three months. Frequent updates can help keep prices aligned with global markets but increase administrative burden and uncertainty for manufacturers.
Can international reference pricing lead to better quality generics?
Not directly. IRP focuses on price, not quality. But companies that want to survive in IRP markets invest in consistent manufacturing, packaging, and bioequivalence testing to build trust. Brands like Sandoz and Hikma use IRP to expand market share by proving their generics are reliable - even at low prices.
Which countries have the most effective IRP systems for generics?
Germany, the Netherlands, and France are often cited as having the most effective systems. Germany uses internal reference pricing with clear rules. The Netherlands combines IRP with tendering. France’s new dynamic system adjusts prices based on real-time sales. All three maintain high availability rates (over 95%) while keeping prices low.
What’s the Bottom Line?
International reference pricing isn’t magic. It doesn’t make drugs cheaper by itself - it just changes how prices are set. Used well, it keeps generics affordable and accessible. Used poorly, it causes shortages and drives manufacturers away. The smartest systems don’t just look at prices. They look at manufacturing complexity, patient needs, and market behavior. And they’re getting smarter - faster.
If you’re in a country using IRP, you’re probably paying less for your pills. But behind that low price is a complex, evolving system - one that balances cost, quality, and supply. The goal isn’t to find the cheapest drug in the world. It’s to find the right drug, at the right price, every time.



Bea Rose
November 27, 2025 AT 07:18Michael Collier
November 27, 2025 AT 21:02Wendy Edwards
November 28, 2025 AT 10:30Jaspreet Kaur
November 29, 2025 AT 13:27Gina Banh
November 30, 2025 AT 14:02Deirdre Wilson
November 30, 2025 AT 21:12Damon Stangherlin
December 1, 2025 AT 01:50Ryan C
December 1, 2025 AT 18:08Dan Rua
December 2, 2025 AT 04:58