Every pill you swallow, every injection you get, every inhaler you use - it’s not magic. It’s chemistry. And if you don’t understand how that chemistry works, you’re flying blind when it comes to safety.
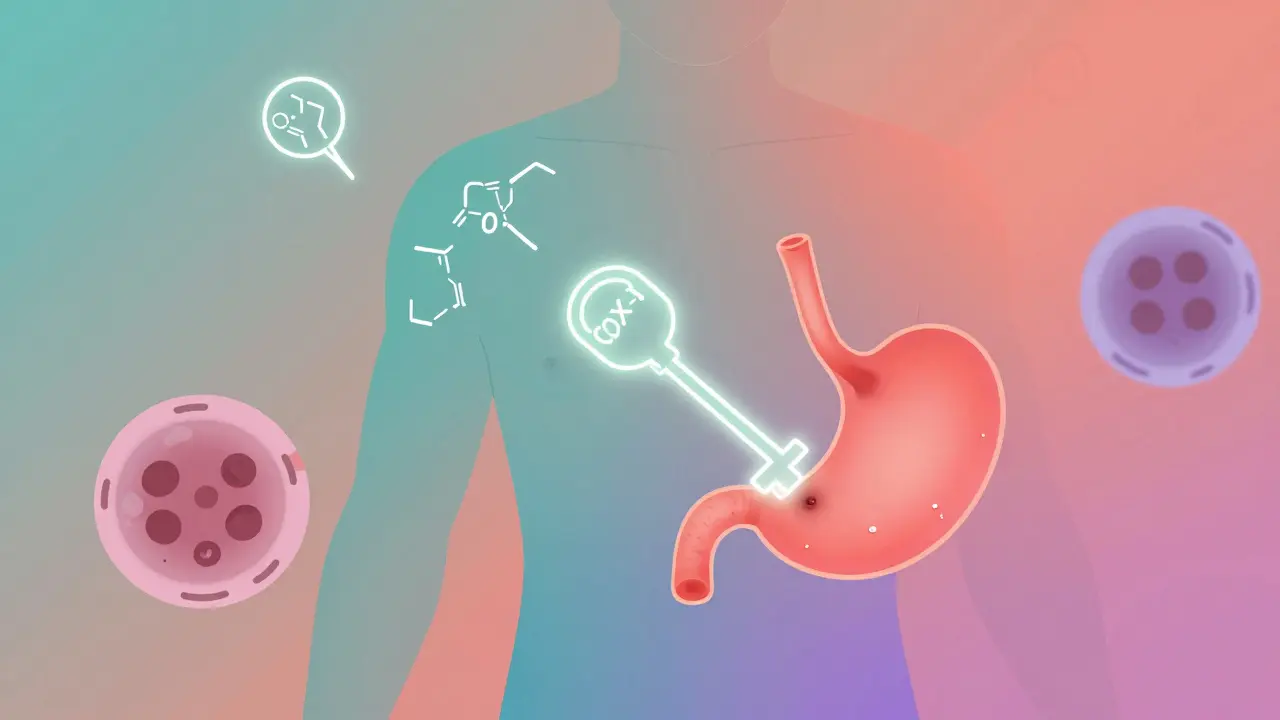
Take aspirin. It doesn’t just ‘take away pain.’ It blocks a specific enzyme called COX-1, which your body uses to make chemicals that cause swelling and pain. That’s its mechanism of action. And because we know this, we can predict what might go wrong. If you take too much, you risk stomach bleeding - because COX-1 also protects your stomach lining. That’s not a random side effect. It’s a direct result of how the drug works.
Most medicines work the same way: they’re tiny chemical keys that fit into specific locks in your body - receptors, enzymes, or transporters. When the key turns, something happens. Maybe it turns a signal on, like insulin helping cells absorb sugar. Maybe it turns a signal off, like antihistamines blocking the histamine that makes your nose run during allergies. Sometimes, the key doesn’t turn the lock at all - it just jams it, preventing the real signal from getting through. That’s what SSRIs like fluoxetine do with serotonin. They block the transporter that recycles serotonin back into nerve cells, leaving more of it floating around to improve mood.
But here’s the catch: your body doesn’t just accept these keys. It fights back. After you swallow a pill, it travels through your stomach and intestines. Some drugs act right there - like laxatives or antacids. Others get absorbed into your blood. Once in your bloodstream, about 95% to 98% of most drugs stick to proteins, like albumin. That’s fine - until another drug comes along and kicks it off. Warfarin, a blood thinner, is 99% protein-bound. If you start taking a sulfonamide antibiotic, it can push warfarin off those proteins. Suddenly, your free warfarin level jumps 20-30%. That’s not a small change. That’s a bleeding risk.
Then there’s the blood-brain barrier. It’s your brain’s bouncer. It lets in glucose and oxygen, but blocks most drugs. That’s why Parkinson’s patients take Sinemet - a combo of levodopa and carbidopa. Levodopa is the actual medicine. Carbidopa doesn’t help the brain - it just stops levodopa from being broken down in the gut and liver first. Without it, you’d need ten times the dose, and it still wouldn’t work well. This is pharmacokinetics: what your body does to the drug. And it’s just as important as pharmacodynamics: what the drug does to your body.
Some drugs are precise. Trastuzumab (Herceptin) only works if your breast cancer cells have too much HER2 protein. Before you get it, you get tested. If you don’t have HER2, the drug won’t help. And you won’t get the side effects - like heart damage - because your body isn’t the target. That’s precision medicine. It’s not guesswork. It’s science.
Other drugs? Not so much. Lithium, used for bipolar disorder, still doesn’t have a clear mechanism. We know it helps stabilize mood, but we don’t know exactly how. That’s why your blood levels have to be checked every few weeks. Too low? No effect. Too high? You get tremors, confusion, even kidney damage. The safe range is tiny: 0.6 to 1.2 mmol/L. One wrong dose, and you’re in the ER.
And then there’s the thalidomide disaster. In the 1950s, it was sold as a safe sleep aid and morning sickness remedy. But one version of the molecule - one mirror-image form - caused severe birth defects. The other version was harmless. We didn’t know then that your body can turn one into the other. Today, we test every single chemical form. That’s why you now see warnings like ‘enantiomer-specific’ on labels.
Understanding how a drug works isn’t just for doctors. It’s for you. On PatientsLikeMe, 68% of users said knowing how their medicine worked helped them spot danger signs earlier. Warfarin users who learned it blocks vitamin K started avoiding huge amounts of kale, spinach, and broccoli - foods packed with 200 to 800 mcg of vitamin K per serving. That’s not a myth. That’s a real interaction. A single large salad can throw your INR off. You don’t need to avoid greens forever - just keep them consistent. That’s safety.
MAO inhibitors for depression? If you eat aged cheese, pickled herring, or cured meats, you risk a hypertensive crisis. Why? Because these foods have tyramine - 1 to 5 mg per ounce. MAO inhibitors stop your body from breaking down tyramine. It builds up, spikes your blood pressure, and can cause a stroke. Patients who understood this didn’t get hospitalized. Those who didn’t? They ended up in the ER.
Statins lower cholesterol by blocking HMG-CoA reductase - the enzyme your liver uses to make cholesterol. But they can also cause muscle pain. If you know that, you don’t ignore it. You tell your doctor. Early reporting cuts the risk of rhabdomyolysis - a rare but life-threatening muscle breakdown - by over 80%. A 2023 Drugs.com analysis showed patients who understood this mechanism were 3.2 times more likely to report muscle pain early.
Even the route matters. Morphine taken orally loses 30% of its strength on the first pass through the liver. Propranolol? Up to 90%. That’s why some drugs are given as patches, injections, or inhalers - to skip the gut and liver entirely. If you take a pill you’re supposed to swallow whole, but you crush it, you’re changing how it’s absorbed. That’s not ‘getting more benefit.’ That’s risking overdose.
Regulators are catching on. The FDA now requires detailed mechanism-of-action data for nearly 90% of new drug applications - up from 62% in 2015. Drugs with clear mechanisms have 34% fewer safety label changes after approval. That’s because we can monitor for specific risks. For example, natalizumab (Tysabri) reduces immune cell entry into the brain - great for MS. But it also raises the risk of a rare brain infection called PML. So doctors must be trained. Patients must sign off. It’s not fear. It’s control.
And it’s getting smarter. The NIH’s All of Us program is collecting genetic data from a million people to see how DNA affects drug response. We now know that 28% of bad reactions are tied to genetic differences in how your body processes drugs. One person might need half a pill. Another might need three. That’s not ‘personalized medicine’ buzzwords. That’s the future - and it’s already here.
So when you pick up a prescription, ask: How does this work? What’s it targeting? What could go wrong because of that? Don’t wait for a side effect to happen. Understand the mechanism before you take the first dose. Your body isn’t a black box. It’s a system - and medicines are tools. Use them right, and they heal. Use them blindly, and they hurt.



Jacob Milano
January 5, 2026 AT 09:00Man, I never realized how wild it is that a tiny molecule can flip a switch in your brain like a light bulb. I used to think meds were just 'magic pills' until my dad started on warfarin and I had to learn why kale was suddenly the enemy. Now I read the insert before I even swallow anything. Mind blown.
Also, the fact that your liver can turn a drug into a different beast? That’s not science-that’s alchemy with a PhD.
saurabh singh
January 5, 2026 AT 22:31Bro, in India we call this 'chemistry ka jadoo'-magic of chemistry. But seriously, this is gold. My uncle took lithium for years and never knew why his blood had to be checked every month. Now I’m sending this to his entire family. Knowledge = power, and power = not ending up in the ER with a shaking hand and a confused look.
Also, thanks for explaining why we don’t just take 10x the dose of levodopa. My aunt tried that once. Bad day.
Dee Humprey
January 6, 2026 AT 12:24Just wanted to say thank you for writing this. As someone who’s been on SSRIs for a decade, this is the first time I’ve understood why I can’t just ‘snap out of it.’ It’s not weakness-it’s a transporter jam. That’s a game-changer for how I talk to my therapist and my mom who still thinks I’m ‘just being dramatic.’
Also, the vitamin K thing with warfarin? I started tracking my greens like a spreadsheet. No more surprise INR spikes. Small changes, huge results.
Allen Ye
January 7, 2026 AT 04:57It’s fascinating how deeply reductionist modern pharmacology has become-we reduce the human organism to a series of molecular interactions, as if the soul were merely an epiphenomenon of receptor binding. But in doing so, we’ve gained an unprecedented ability to intervene with precision. The tragedy of thalidomide wasn’t just ignorance-it was the hubris of assuming symmetry in nature is always benign. The mirror-image molecule wasn’t evil; it was simply misunderstood. And isn’t that the story of every great scientific leap? We don’t conquer nature-we learn to speak its language, one chiral center at a time.
Perhaps the most radical act isn’t taking the pill, but asking why it works. That question, more than any drug, is the true medicine.
mark etang
January 8, 2026 AT 19:02Thank you for this meticulously researched and clinically significant exposition. The integration of pharmacokinetic and pharmacodynamic principles with real-world patient outcomes demonstrates a profound understanding of translational medicine. Regulatory evolution, as evidenced by the FDA’s increased requirement for mechanism-of-action data, reflects a necessary paradigm shift toward evidence-based safety protocols. This level of scientific literacy among patients is not merely beneficial-it is imperative for public health.
jigisha Patel
January 10, 2026 AT 17:43Wow. So you’re telling me the reason people don’t understand their meds is because they’re too lazy to read the 50-page FDA monograph? Let me guess-you also think people should memorize the entire Merck Manual before taking ibuprofen? This isn’t medicine, it’s a cult of pharmaceutical literacy. Most people don’t need to know about COX-1. They need a doctor who knows. And if you’re taking 10 different pills and reading up on each mechanism, you’re not empowered-you’re neurotic.
Jason Stafford
January 11, 2026 AT 01:14They don’t want you to know this. They’re hiding the truth. Every drug is designed to keep you dependent. COX-1? That’s a lie. The real reason aspirin causes bleeding is because Big Pharma doesn’t want you to heal naturally. They profit from your stomach ulcers. The blood-brain barrier? It’s not a barrier-it’s a filter they control. They only let in what keeps you docile. And the genetic stuff? They’re already testing this on you through vaccines and fluoridated water. Wake up. The pills aren’t healing you-they’re programming you.
PS: I’ve been off all meds for 3 years. I eat turmeric and breathe deep. My INR? Perfect. Coincidence? I think not.
Justin Lowans
January 12, 2026 AT 11:05This is one of the clearest, most thoughtful explanations of pharmacology I’ve ever read. The way you tied mechanism to real-life outcomes-warfarin and kale, SSRIs and serotonin transporters-it turns abstract science into something you can hold in your hands. I’ve shared this with my book club. We’re all going to start asking our doctors: ‘How does this work?’ Not ‘Is it safe?’-but ‘How?’ That shift alone could save lives.
Michael Rudge
January 13, 2026 AT 22:22Oh wow. So you’re telling me that people who don’t know their drug’s mechanism are just… dumb? Congrats. You’ve just written a 1000-word essay to say ‘read the label.’ How revolutionary. I’m sure the 80-year-old with dementia who takes 12 pills a day is just too lazy to Google ‘pharmacokinetics.’
Also, ‘ask your doctor’ is the most overused phrase in medicine. It’s the medical equivalent of ‘just be positive.’
Rory Corrigan
January 14, 2026 AT 13:05Everything is energy, man. Even pills. That COX-1 enzyme? It’s not just a protein-it’s a vibration. Your body knows when you’re aligned. The real medicine isn’t in the pill-it’s in the intention behind taking it. I took my fluoxetine once with a crystal on my chest. Mood lifted. Coincidence? Maybe. Or maybe the universe just nodded.
Also, kale is a portal. I only eat it under a full moon. Works better.
Stephen Craig
January 16, 2026 AT 09:40Understanding the mechanism helps you notice side effects early. That’s it.
Connor Hale
January 17, 2026 AT 02:24Interesting how we’ve gone from ‘trust your doctor’ to ‘understand every molecular interaction’ in just a few decades. I wonder if we’re better off now-or just more anxious. Either way, I’m glad someone took the time to explain this without sounding like a textbook. It’s rare to see science this clear without the hype.
Also, I still don’t know how lithium works. And honestly? I’m okay with that.