Think about this: you take a generic pill every day-maybe metformin for diabetes, or levothyroxine for your thyroid. It’s cheap, effective, and you’ve been on it for years. Now imagine you travel to Canada, Switzerland, or India, and try to buy the exact same pill. The name is the same. The active ingredient is identical. But the price? It could be one-tenth-or ten times-what you pay at home. Why does this happen?
Same Drug, Different Worlds
Generic drugs are supposed to be the same as brand-name drugs. They contain the same active ingredient, work the same way, and meet the same safety standards. But globally, their availability, price, and even how often they’re prescribed vary wildly. In the United Kingdom, 83% of all prescriptions are filled with generics. In Switzerland, it’s just 17%. That’s not a difference in medical need-it’s a difference in policy, culture, and market structure. The U.S. leads the world in generic prescription volume, with over 90% of prescriptions filled with generics as of 2023. But here’s the twist: the U.S. also pays more for drugs than any other country. Americans pay 2.78 times more for the same medications than people in other OECD nations. So even though you’re getting the generic version, you’re still paying more than someone in Germany or the UK for the exact same pill.Who Makes Your Pills-and Where?
Most of the generic drugs you take don’t come from local pharmacies. They’re made in factories thousands of miles away. India produces about 20% of the world’s generic drugs and supplies 40% of the generics used in the U.S. As of 2023, India had over 750 manufacturing facilities approved by the U.S. FDA. China is catching up fast-its FDA-approved facilities jumped from 12 in 2010 to 187 in 2023. But quality isn’t guaranteed just because a drug is approved. A 2023 study from Ohio State University found that generics made in India were linked to 54% more severe adverse events-including hospitalizations and deaths-compared to the same drugs made in the U.S. This isn’t about fraud. It’s about cost pressure. When a drug has dozens of manufacturers competing to sell it for pennies, some cut corners on raw materials, testing, or production controls. That’s why you might hear stories of patients switching from a U.S.-made generic to an Indian version and suddenly experiencing new side effects.Why Some Countries Use Generics More Than Others
It’s not just about cost. It’s about rules. In the UK, pharmacists are required to substitute brand-name drugs with generics unless the doctor says no. In Germany, doctors are paid bonuses for prescribing generics. In Switzerland, doctors rarely prescribe them. Why? Because Swiss insurers reimburse brand-name drugs at the same rate as generics, so there’s no financial incentive to switch. In Southern Europe, generic use is low. Italy and Greece hover around 20%. In contrast, the Netherlands and Germany are above 70%. The difference? Policy. Countries that force substitution or reward prescribing generics see faster adoption. Countries that leave it up to doctors and patients? It takes years-sometimes decades-for generics to catch on.
The Price Puzzle: Why Is the Same Pill So Expensive in Some Places?
Let’s say you’re buying a generic version of the blood pressure drug amlodipine. In the U.S., it might cost $4 for a 30-day supply. In the UK, it’s $0.50. In Switzerland, it’s $12. All the same pill. All made in the same factory, probably in India. The reason? Pricing systems. The U.S. doesn’t negotiate drug prices like other countries. Medicare can’t negotiate. Private insurers negotiate separately. Pharmacies mark up prices. Middlemen take cuts. Meanwhile, countries like the UK and Germany set price caps. They look at what other countries pay and say, “This is fair.” Even within Europe, prices vary wildly. Switzerland’s manufacturer prices are more than six times higher than the UK’s for the same generic. That’s why people in the U.S. and Canada buy drugs online from India or Mexico-they’re not breaking the law to be risky. They’re doing it because it’s the only way to afford their meds.Supply Chains Are Fragile
During the pandemic, India temporarily stopped exporting 26 key generic ingredients. Overnight, hospitals in 22 countries ran low on antibiotics, antifungals, and blood pressure drugs. Why? Because the world depends on just a few factories to make the same drugs. If one facility fails an FDA inspection, it can cause a shortage across the entire U.S. market. In 2023, the FDA recorded 147 generic drug shortages. Two-thirds were caused by manufacturing quality issues-mostly at single-source plants in India and China. That’s the risk of hyper-concentration. When 80% of your generic pills come from just two countries, a regulatory crackdown, a factory fire, or a political dispute can leave millions without their medicine.Regulation Is a Patchwork
The U.S. FDA requires generics to be bioequivalent-meaning they must absorb into the bloodstream within 80-125% of the brand-name drug’s rate. The European Medicines Agency uses similar standards. But here’s the catch: the FDA inspects foreign factories with little warning. Europe often gives manufacturers months of notice before inspections. That means factories can clean up before inspectors arrive. Dr. Ameet Sarpatwari from Harvard pointed out in JAMA that this creates a dangerous blind spot. A generic drug approved in Europe might be perfectly safe there, but if the same factory ships to the U.S. without the same level of oversight, the quality can drop. That’s why some patients report different side effects when they switch from a U.S.-made generic to a European or Indian one-even though the label says it’s the same.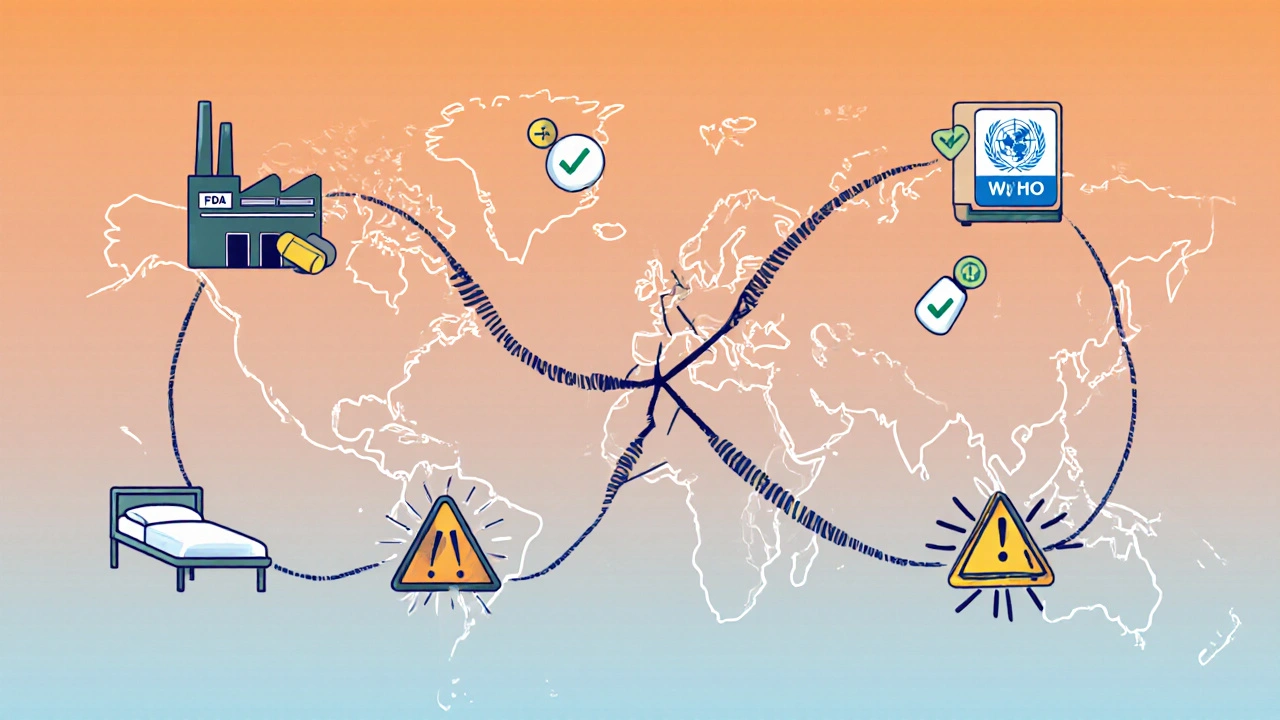
What’s Changing?
There’s movement. The U.S. Inflation Reduction Act of 2022 includes funding to speed up FDA reviews of generics by 30%. The European Commission wants 80% generic use across all member states by 2030. The WHO released a new global benchmarking tool in 2024 to help countries improve their generic drug quality checks. But big barriers remain. Drugmakers still file dozens of minor patents-called “evergreening”-to delay generic entry. For 12 top-selling drugs between 2015 and 2022, companies filed over 1,200 supplemental patents to keep generics off the market longer. Meanwhile, AI is starting to help. By 2030, AI could cut the time it takes to develop a generic drug from 3-5 years to just 18-24 months. That could mean more competition, lower prices, and fewer shortages-if regulators keep up.What This Means for You
If you’re on a generic drug, here’s what you should know:- Switching between manufacturers-even if they’re both “generic”-can sometimes change how you feel. If you notice new side effects after a refill, talk to your pharmacist.
- Prices vary by pharmacy, even in the same city. Use tools like GoodRx to compare prices before you pay.
- Buying from international pharmacies can save money, but only use ones that are verified (like PharmacyChecker-approved sites). Unregulated sources carry real risk.
- Ask your doctor if your drug has multiple generic versions. Sometimes, one manufacturer’s version works better for you than another’s-even if they’re technically identical.
What’s Next?
The global generic market is worth $450 billion-and growing. But it’s built on a shaky foundation: a few factories, inconsistent rules, and a system that rewards low cost over reliability. The solution isn’t to stop using generics. It’s to fix how they’re made, regulated, and priced. Countries that treat generics like essential public goods-like the UK and Germany-get better outcomes. Countries that treat them like commodities-like the U.S.-get higher prices and more shortages. The future of affordable medicine doesn’t lie in brand-name drugs. It lies in fixing the system that delivers the ones we already have.Why are generic drugs cheaper than brand-name drugs?
Generic drugs are cheaper because they don’t need to repeat expensive clinical trials. Once a brand-name drug’s patent expires, other companies can copy the formula. They only need to prove the drug works the same way in the body-called bioequivalence. That cuts development costs by 80-90%. They also don’t spend millions on advertising. That’s why a generic version of a drug can cost 80-95% less than the brand-name version.
Are generic drugs as safe and effective as brand-name drugs?
Yes, by law. In the U.S., Canada, the EU, and most developed countries, generics must meet the same quality, strength, purity, and performance standards as brand-name drugs. The FDA requires them to be bioequivalent. But safety depends on manufacturing. A 2023 study found that generics made in India had 54% more severe adverse events than those made in the U.S., likely due to cost-cutting in production. So while the active ingredient is the same, the quality of the pill can vary by manufacturer and country.
Why do prices for the same generic drug differ so much between countries?
It’s because of how each country sets drug prices. In the U.S., prices are set by private negotiations between drugmakers, insurers, and pharmacies-with no government price control. In the UK, Germany, and Canada, the government negotiates or sets price caps. In Switzerland, brand-name drugs are reimbursed at the same rate as generics, so there’s no incentive to switch. That’s why a $4 generic pill in the U.S. can cost $0.50 in the UK and $12 in Switzerland-all the same drug, same factory.
Can I trust generics made in India or China?
Many are safe and effective. India produces 40% of U.S. generics and has over 750 FDA-approved facilities. China is rapidly expanding its output. But quality control varies. A 2023 study found Indian-made generics had higher rates of serious side effects. If you’re switching to a new generic, especially one from a new manufacturer, watch for changes in how you feel. Use verified online pharmacies (like PharmacyChecker-approved sites) if buying internationally. Avoid unregulated sellers.
Why do some doctors refuse to prescribe generics?
Some doctors believe brand-name drugs are more reliable, especially for drugs with narrow therapeutic windows-like blood thinners or seizure meds. Others are influenced by patient preference or reimbursement rules. In Switzerland, insurers pay the same for brand and generic, so doctors have no reason to switch. In the UK, doctors are paid more for prescribing generics. It’s not about medical science-it’s about incentives, habits, and policy.
What’s being done to fix global generic drug problems?
The U.S. Inflation Reduction Act is speeding up FDA reviews of generics by 30% and increasing inspections of foreign factories. The WHO launched a new global benchmarking tool in 2024 to help countries improve quality control. The European Union wants 80% generic use by 2030. AI is also being used to shorten development time from 5 years to under 2 years. But progress is slow. The biggest obstacle? Regulatory fragmentation-each country has its own rules, making it hard to create a truly global, reliable supply.




Karandeep Singh
December 1, 2025 AT 02:06India makes 40% of US generics and you act like we're poison factories
ariel nicholas
December 1, 2025 AT 14:57Of course the U.S. pays more-because we don't let socialist bureaucrats dictate prices! You want cheap drugs? Move to Venezuela. Or better yet-stop whining and get a second job.
Debbie Naquin
December 2, 2025 AT 10:00The structural asymmetry in global pharmaceutical governance creates a paradox: bioequivalence is a pharmacokinetic metric, yet quality assurance is a geopolitical artifact. The FDA's surprise inspections are performative compliance theater while EMA's advance notice protocols incentivize cosmetic remediation. The result? A global supply chain where regulatory arbitrage supersedes therapeutic fidelity.
Amber-Lynn Quinata
December 3, 2025 AT 11:22I just switched my generic metformin and now I'm dizzy and nauseous 😔 I told my pharmacist but they said 'it's the same thing!' NO IT'S NOT. I'm not crazy. This is dangerous. People are dying because of this. 😡
Kelly Essenpreis
December 5, 2025 AT 10:13Why are we even talking about this like it's a mystery? America pays more because we're the suckers who fund R&D for the whole world. India and China free ride and then act like they're saints. Wake up people
elizabeth muzichuk
December 6, 2025 AT 10:31My cousin died because of a bad generic. It was made in India. The label said it was the same. It wasn't. I've been screaming about this for years and no one listens. Now my mom won't take her pills anymore. This isn't just about money. It's about people dying quietly while politicians sip lattes.
Rachel Stanton
December 7, 2025 AT 18:25For anyone on generics: if you notice new side effects after a refill, talk to your pharmacist immediately. Different manufacturers use different fillers and binders-those aren't inert. They can affect absorption. Also, GoodRx is your friend. And yes, some versions work better for you than others-even if they're 'identical.' Your body knows.
Lauryn Smith
December 8, 2025 AT 00:20I get it. It's scary to think your medicine might be different depending on where it came from. But most generics are fine. The key is to stick with one brand if it works for you. And if you're worried, ask your doctor to write 'do not substitute' on the script. You're not being difficult-you're being smart.
Bonnie Youn
December 8, 2025 AT 21:30STOP PANICKING. India has 750 FDA-approved plants. That's not a bug-it's a feature. We're getting life-saving meds for pennies. If you don't like it, go back to paying $400 for brand-name insulin. I'm not sorry. People need this. Let's fix the system not panic about it
Charlotte Collins
December 9, 2025 AT 01:01Let’s be brutally honest: the U.S. pharmaceutical system is a carnival of rent-seeking, patent trolling, and middleman exploitation. The real scandal isn’t that Indian generics have more side effects-it’s that we’ve outsourced our moral responsibility to factories that operate under the bare minimum of oversight. We didn’t lose control of drug quality-we surrendered it willingly for profit.
Margaret Stearns
December 10, 2025 AT 16:04i think we need to talk about this more. i switched generics last month and my blood pressure went crazy. i didn't know it could happen. i'm not techy but i looked up my pill and found out it was made in china. now i only buy the ones made in the usa. simple as that.
amit kuamr
December 11, 2025 AT 15:55Why are Americans so shocked? We import everything. Food. Electronics. Medicine. If you want quality pay more. If you want cheap accept risk. This is basic economics. Stop pretending the world owes you safety without cost
Scotia Corley
December 12, 2025 AT 10:36It is a matter of public record that the United States maintains the most rigorous pharmacovigilance and manufacturing oversight in the world. The assertion that foreign-manufactured generics are inherently inferior is not supported by empirical evidence on a macro scale. Rather, it reflects a cognitive bias toward domestic production.
Alexander Williams
December 13, 2025 AT 10:28The entire framework is a rent extraction machine. Evergreening patents, patent thickets, AND then outsourcing production to jurisdictions with weaker enforcement? That's not capitalism. That's feudalism with FDA logos. The system doesn't need reform-it needs dismantling. AI won't fix it. Only systemic disruption will.