When a life-saving drug disappears from pharmacy shelves, it’s rarely because of a sudden spike in demand. More often, it’s the result of a broken supply chain-something most people never see until it’s too late. In 2025, drug shortages still plague hospitals and clinics across the U.S., with over 200 essential medicines, including antibiotics, cancer treatments, and injectable anesthetics, regularly unavailable. The problem isn’t getting better. It’s getting more complex. And the solution isn’t about rushing to stockpile pills. It’s about rebuilding the entire system that gets those pills from factory to patient.
Why the Current System Is Fragile
For decades, pharmaceutical companies chased efficiency above all else. They moved production overseas to cut costs, relying on just-in-time manufacturing and single-source suppliers. It worked-until it didn’t. The COVID-19 pandemic exposed how thin the margins were. When factories in China shut down, or shipping lanes got blocked, the ripple effects hit U.S. hospitals hard. Today, about 80% of the active ingredients in U.S. medications come from abroad, mostly from China and India. That’s not just a number-it’s a vulnerability. Take sterile injectables. These are critical for surgeries, emergency care, and cancer treatment. Yet, the U.S. produces only 12% of them domestically. One supplier goes offline, and hospitals scramble. That’s not resilience. That’s risk on autopilot.What Resilience Really Means
Resilience isn’t about making everything in America. It’s about making sure disruptions don’t cause collapse. The U.S. Department of Health and Human Services defines it clearly: the ability to anticipate, prepare for, respond to, and recover from disruptions while keeping critical drugs flowing. That’s the goal. And it’s achievable-but only if you change how you think about supply chains. Think of it like a flood system. You don’t build one giant dam and hope it holds. You build levees, drainage channels, backup pumps, and early warning systems. Same here. Resilience means having multiple ways to get the same drug to patients, even when one path fails.Three Core Strategies That Work
Leading companies and regulators have identified three proven strategies that reduce the chance of shortages:- Buffer stock for critical drugs-keeping 60 to 90 days of inventory for essential medicines like epinephrine, insulin, and antibiotics. This isn’t waste. It’s insurance. Hospitals that maintain these reserves report 70% fewer emergency shortages.
- Dual-sourcing critical components-never relying on just one supplier for active pharmaceutical ingredients (APIs). Top performers now dual-source 70-80% of their most important inputs. If one plant shuts down, another can ramp up within weeks, not months.
- Regional manufacturing networks-building production hubs in North America, Europe, and Asia-Pacific. This isn’t about bringing everything home. It’s about having at least three geographic zones producing the same drug. That way, a flood in India or a labor strike in Germany won’t stop U.S. access.
Technology Is Changing the Game
Old-school batch manufacturing-where drugs are made in large, slow batches-is being replaced by continuous manufacturing. It’s faster, cheaper to run, and uses 30-40% less space. A continuous manufacturing facility can produce the same amount of API in a warehouse-sized building that used to need a football-field-sized plant. And it’s more flexible. If demand spikes, you just turn up the flow rate. But it’s expensive. Setting up one facility costs $50-150 million-three to five times more than a traditional plant. That’s why only 12 such facilities have FDA approval as of mid-2025, compared to over 10,000 batch plants. AI is helping too. Machine learning models now predict supply disruptions 60-90 days in advance with 85-90% accuracy. They track weather patterns, port delays, political unrest, and even supplier financial health. One major pharma company used this to reroute a shipment of insulin just before a cyclone hit a key port in Mumbai. No shortage. No panic. Blockchain is cutting down counterfeit drugs-once a hidden threat, now a growing risk. Pilots show a 70-75% drop in fake meds when every step of the supply chain is digitally tracked. That’s not just about safety. It’s about trust.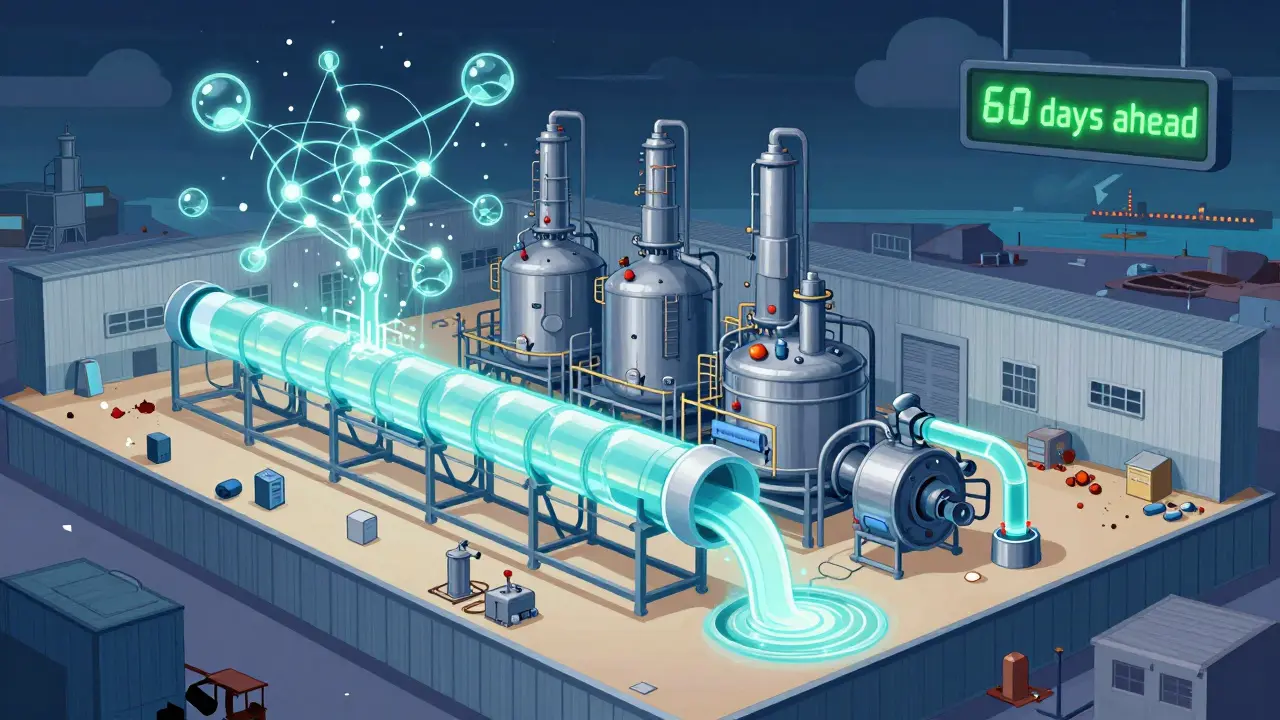
Government Action Is Accelerating
The federal government is stepping in-not with mandates, but with incentives. In August 2025, President Trump signed an executive order to fill the Strategic Active Pharmaceutical Ingredients Reserve. The goal? By 2027, have 90-day stockpiles of 150 essential medicines ready to deploy in emergencies. The 2023 CHIPS and Science Act allocated $1.2 billion to build domestic manufacturing capacity. An additional $800 million was proposed in the 2025 budget. This isn’t just about making drugs. It’s about making sure the U.S. can respond to bioterrorism, pandemics, or cyberattacks on infrastructure. The FDA is also adapting. Approval times for continuous manufacturing facilities have dropped from 2-3 years to just 12-18 months. That’s huge. It means innovation can move faster.Costs Are Real-But So Are the Consequences
Yes, building resilience costs more. Companies now spend 8-12% more on their cost of goods sold to maintain buffer stocks, dual sources, and new tech. But here’s what they’re avoiding: $14.7 million in lost revenue per major disruption, according to ZS Associates. That’s not hypothetical. It’s real money from delayed treatments, emergency imports, and lost patient trust. And the cost of inaction? Hospitals cancel surgeries. Cancer patients wait weeks for chemo. Elderly patients risk death from untreated infections. No amount of savings justifies that.Who’s Doing It Right?
Large pharmaceutical companies with over $10 billion in revenue are leading the way. 85% have full resilience programs. Mid-sized firms? Only 42%. Small ones? Just 18%. Why the gap? It’s not just money. It’s leadership. Companies where the CEO owns supply chain risk see 3.2 times more success in implementation. One medtech company partnered with ZS Associates to map all 15 tiers of its supplier network. They found a single supplier in China controlled 90% of a key API for a heart drug. Within six months, they qualified a second supplier in Spain and started stockpiling. No shortage since.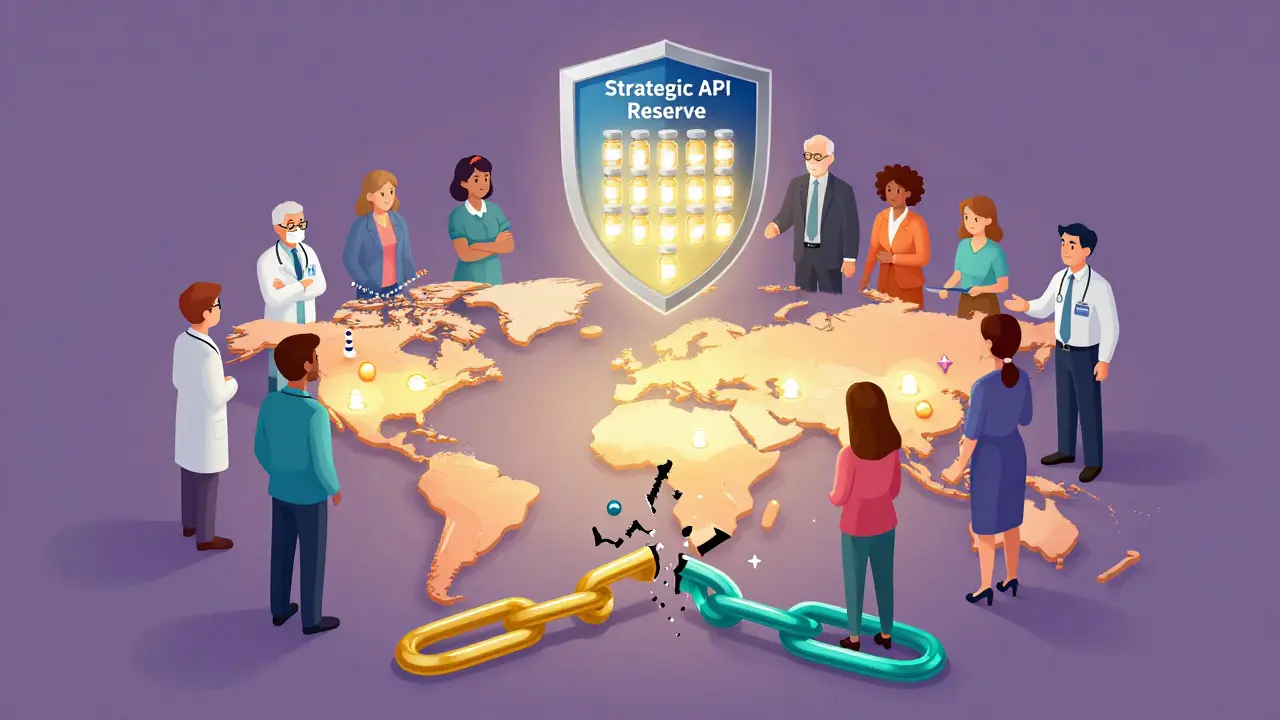
The Biggest Mistake: Over-Reliance on One Solution
Some argue we should bring all manufacturing back to the U.S. That’s not realistic. It would raise drug prices by 20-30% and still leave gaps. The National Academies of Sciences warns that single-sourcing domestically creates new risks. If one U.S. plant fails, you’re back to square one. Others think tariffs will fix it. They won’t. Tariffs might make foreign production less attractive, but they don’t build factories, train workers, or create regulatory expertise. The CSIS report says it plainly: “Deploying tariffs to onshore all aspects of the pharmaceutical supply chain is infeasible.” The right path? A mix. Strategic domestic capacity for critical drugs. Regional hubs for others. Global diversity for non-critical items. And technology to tie it all together.What You Can Do-Even If You’re Not a CEO
You don’t need to run a multinational to help. If you’re a pharmacist, ask your distributor: “Do you have dual sources for these high-risk drugs?” If you’re a hospital administrator, push for buffer stock policies. If you’re a policymaker, support funding for continuous manufacturing grants. Patients can also speak up. When a drug is out of stock, report it to the FDA’s Drug Shortages website. These reports trigger alerts and help prioritize response.The Future Is Already Here
By 2027, 45-50% of new drug manufacturing will use continuous processes. By 2030, 65-70% of U.S. pharmaceutical needs will come from regional networks, not just one global hub. The shift isn’t coming. It’s happening. The goal isn’t perfection. It’s reliability. A world where a child with asthma gets their inhaler. Where a cancer patient gets their next dose on time. Where no one dies because a shipment got stuck in a port. That’s not a dream. It’s a design choice. And we’re finally choosing wisely.What causes drug shortages in the U.S.?
Drug shortages in the U.S. are most often caused by disruptions in the global supply of active pharmaceutical ingredients (APIs), which are primarily manufactured in China and India. Other causes include manufacturing quality issues, regulatory delays, single-source suppliers, and logistical breakdowns like port closures or shipping delays. Natural disasters, political instability, and labor strikes can also interrupt production.
How many drugs are currently in shortage?
As of 2025, over 200 essential medications remain in shortage across the U.S., including antibiotics, chemotherapy drugs, anesthetics, and injectables. The FDA tracks these shortages and updates its public list monthly. Sterile injectables and generic drugs are the most affected categories.
What is the Strategic Active Pharmaceutical Ingredients Reserve?
The Strategic Active Pharmaceutical Ingredients Reserve is a U.S. government initiative launched in August 2025 to stockpile critical APIs for 150 essential medicines. The goal is to maintain a 90-day supply of these ingredients by 2027, so they can be rapidly deployed during emergencies like pandemics, natural disasters, or geopolitical disruptions.
Why isn’t all drug manufacturing done in the U.S.?
Manufacturing drugs domestically is far more expensive-up to 30% higher in costs-due to labor, regulatory, and infrastructure expenses. Many APIs require specialized chemical processes that were outsourced decades ago for cost efficiency. Building new U.S. facilities takes years and billions in investment. The focus now is on strategic domestic capacity for critical drugs, not full onshoring.
How does continuous manufacturing improve supply chain resilience?
Continuous manufacturing produces drugs in a constant, automated flow instead of in batches. This reduces production time by up to 50%, cuts facility size by 30-40%, lowers energy use by 20-25%, and reduces waste. It’s also more flexible-output can be adjusted quickly to meet demand spikes. These advantages make it easier to respond to disruptions and scale production without massive capital overhauls.
Can AI really predict drug shortages before they happen?
Yes. AI models now analyze global data-including port delays, supplier financial health, weather patterns, and political events-to predict disruptions with 85-90% accuracy up to 90 days in advance. Companies using these tools have reduced unplanned shortages by 40-60% by rerouting supply chains or adjusting production schedules early.
What’s the difference between dual-sourcing and single-sourcing?
Single-sourcing means relying on one supplier for a critical drug ingredient. If that supplier has a problem, you lose access. Dual-sourcing means having two or more qualified suppliers for the same ingredient. This ensures continuity if one fails. Top pharmaceutical companies now dual-source 70-80% of their most critical APIs to avoid disruptions.
How can patients help reduce drug shortages?
Patients can report drug shortages to the FDA’s Drug Shortages website. These reports help the agency prioritize responses and identify emerging issues. Patients can also ask pharmacists if alternative medications are available and avoid hoarding prescriptions, which worsens shortages for others.




Dorine Anthony
December 19, 2025 AT 06:56Been a pharmacist for 12 years and I can tell you - the shortages aren’t random. They’re predictable. I’ve watched the same 5 drugs vanish every winter like clockwork. Buffer stocks? We’ve been begging for them. Hospitals keep saying ‘budget constraints.’ Meanwhile, nurses are calling around to 3 different distributors just to get epinephrine for a kid having anaphylaxis. It’s not a crisis. It’s negligence.
James Stearns
December 19, 2025 AT 08:07It is with profound regret that I must observe the lamentable state of pharmaceutical logistics in the United States. The systemic abandonment of domestic manufacturing capacity, driven by short-term fiscal calculus, has rendered our healthcare infrastructure alarmingly vulnerable. One must ask: Is the pursuit of marginal cost reduction worth the potential loss of human life? The answer, in my estimation, is not merely affirmative - it is morally indefensible.
Aadil Munshi
December 21, 2025 AT 01:53Bro, you think this is new? India’s been making 70% of your APIs since the 90s because your folks didn’t wanna get their hands dirty with chemistry. Now you’re shocked? 😅 We built the infrastructure, you built the profit margins. And now you want to ‘reshore’? Good luck finding 10,000 trained chemists who’ll work for $18/hr. Meanwhile, your ‘Strategic Reserve’ is just a fancy way of saying ‘we’re gonna hoard and charge more.’
Adrienne Dagg
December 22, 2025 AT 14:17AI predicting shortages?? 🤯 That’s wild. I just reported my insulin being out last week on the FDA site and got an email back in 2 days. Like… they actually read it?? 😭 People, PLEASE report shortages. It’s not just ‘inconvenient’ - it’s life or death. And yeah, dual sourcing? YES. My hospital just switched to a backup API for vancomycin and we haven’t had a hiccup since. 🙌
pascal pantel
December 24, 2025 AT 03:31Let’s cut through the buzzword bingo. Continuous manufacturing isn’t a magic bullet - it’s capital-intensive, regulatory hell, and only viable for high-volume, low-complexity molecules. You’re talking about 12 FDA-approved facilities? That’s less than 0.1% of global capacity. And AI? It’s trained on historical data from a broken system. If the root cause is supplier concentration, no algorithm fixes that unless you force diversification - which no one’s willing to do because it eats margins. This is theater with a white coat.
Gloria Parraz
December 24, 2025 AT 06:50I work in a rural ER. We lost three weeks of lidocaine last year. Three weeks. No anesthesia for lacerations. No pain control for MI patients. We used saline flushes to dilute what little we had left. People think this is about ‘supply chains’ - it’s not. It’s about whether we value human life enough to pay for it. This isn’t policy. It’s survival. And we’re failing.
Nicole Rutherford
December 25, 2025 AT 07:38Oh please. ‘Strategic Reserve’? More like a PR stunt. You think the government’s gonna release those APIs fast enough when a crisis hits? Or are they gonna sit on them until the media stops caring? And don’t get me started on ‘regional hubs’ - that’s just outsourcing with a map. You’re still relying on the same corrupt, opaque global system. This whole thing is just rich people pretending they care while they keep paying CEOs $50M to ‘optimize.’
Mark Able
December 26, 2025 AT 03:00My cousin works at a small pharma co. They dual-sourced their API last year. Cost them $2M. Got a 15% price increase. Lost 3 clients. Now they’re shutting down. So yeah - resilience is great. But who pays? The patients? The small manufacturers? The nurses? The system’s rigged. You can’t fix it with tech and slogans. You need to break the profit model.